Ap Chem Periodic Table

The periodic table is the cornerstone of chemistry, a systematic arrangement of elements that reveals profound insights into their properties, behaviors, and relationships. For AP Chemistry students, mastering this tool is not just about memorization but understanding the underlying principles that govern elemental characteristics. This article delves into the periodic table’s structure, trends, and applications, providing a comprehensive guide tailored to advanced learners.
The Architecture of the Periodic Table

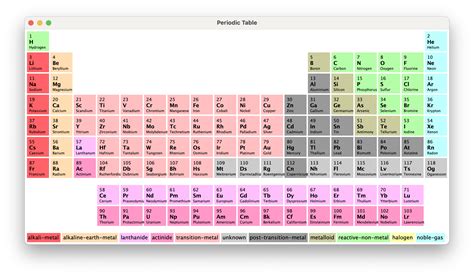
The periodic table is organized by atomic number, the number of protons in an atom’s nucleus. Elements are arranged in rows called periods and columns known as groups or families. This structure highlights recurring patterns in elemental properties, a phenomenon known as periodicity.
Key Insight: The modern periodic table is a result of Dmitri Mendeleev's 1869 arrangement, which predicted the existence of then-undiscovered elements based on gaps in the pattern.
Groups and Periods: A Closer Look
- Groups (1-18): Vertical columns where elements share similar chemical properties due to identical valence electron configurations. For example, Group 1 (alkali metals) are highly reactive due to their single valence electron.
- Periods (1-7): Horizontal rows that indicate the number of electron shells. As you move across a period, the number of valence electrons increases, leading to a shift in properties from metallic to nonmetallic.
Periodic Trends: Predicting Elemental Behavior

Understanding periodic trends is crucial for predicting how elements will react and interact. These trends are directly related to the electronic structure of atoms.
Atomic Radius
- Trend Across a Period: Decreases from left to right due to increasing nuclear charge, pulling electrons closer to the nucleus.
- Trend Down a Group: Increases due to the addition of electron shells, which are further from the nucleus.
Ionization Energy
- Across a Period: Generally increases as the nuclear charge increases, making it harder to remove an electron.
- Down a Group: Decreases because the valence electrons are farther from the nucleus, requiring less energy to remove.
Electronegativity
"Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond."
- Trend Across a Period: Increases from left to right, peaking at fluorine, the most electronegative element.
- Trend Down a Group: Decreases as the atomic radius increases, reducing the nucleus’s pull on electrons.
Metallic vs. Nonmetallic Character
The periodic table visually distinguishes metals from nonmetals, with a stair-step line separating the two. Metals are typically found on the left side and are characterized by their ability to conduct electricity, malleability, and ductility. Nonmetals, on the right, are generally poor conductors and are more brittle.
Key Takeaway: The transition metals, found in the middle, exhibit properties intermediate between metals and nonmetals, often forming colored compounds and multiple oxidation states.
Practical Applications and Real-World Connections
The periodic table is not just an academic exercise; it has profound implications in various fields, from materials science to medicine.
Case Study: Semiconductor Industry
Elements like silicon (Group 14) and germanium are crucial in the semiconductor industry due to their unique ability to conduct electricity under specific conditions. This property is directly tied to their position in the periodic table and their electron configurations.
Environmental Chemistry
Understanding the reactivity of elements helps in addressing environmental issues. For instance, the high reactivity of alkali metals (Group 1) with water makes them unsuitable for exposure to moisture, a critical consideration in storage and handling.
Advanced Concepts: Electron Configurations and the Aufbau Principle
The Aufbau principle, Pauli exclusion principle, and Hund’s rule govern the filling of electron orbitals, providing a deeper understanding of the periodic table’s structure.
Aufbau Principle: Electrons fill orbitals starting from the lowest energy level. For example, the electron configuration of sodium (Na) is 1s² 2s² 2p⁶ 3s¹, reflecting its position in Group 1, Period 3.
FAQ Section
Why do elements in the same group have similar properties?
+Elements in the same group have the same number of valence electrons, which are responsible for chemical bonding and reactions. This similarity in electron configuration leads to analogous chemical behaviors.
How does the periodic table help in predicting chemical reactions?
+By understanding periodic trends, such as electronegativity and ionization energy, chemists can predict the likelihood of certain reactions. For example, highly electronegative elements tend to form ionic bonds with metals.
What are lanthanides and actinides, and why are they placed separately?
+Lanthanides and actinides are series of elements with similar properties, often called inner transition metals. They are placed separately at the bottom of the table to maintain its compact structure, as their inclusion in the main body would make the table excessively wide.
How does atomic radius affect chemical reactivity?
+Larger atomic radii generally result in lower reactivity because the valence electrons are farther from the nucleus, reducing the effective nuclear charge and making it easier to lose or share electrons.
What role does the periodic table play in material science?
+Material scientists use the periodic table to select elements with desired properties, such as conductivity, strength, or corrosion resistance, for developing new materials like alloys, semiconductors, and composites.
Conclusion: The Periodic Table as a Living Document
The periodic table is more than a static chart; it is a dynamic tool that continues to evolve with scientific discoveries. For AP Chemistry students, it is a gateway to understanding the fundamental principles of chemistry and their applications in the real world. By mastering its intricacies, learners can predict elemental behaviors, comprehend chemical reactions, and innovate across various disciplines.
As research progresses, the periodic table may expand with new elements, further enriching our understanding of the chemical universe. Its enduring relevance underscores the importance of a deep, analytical approach to this essential tool in chemistry education.

