Ch3oh Lewis Structure
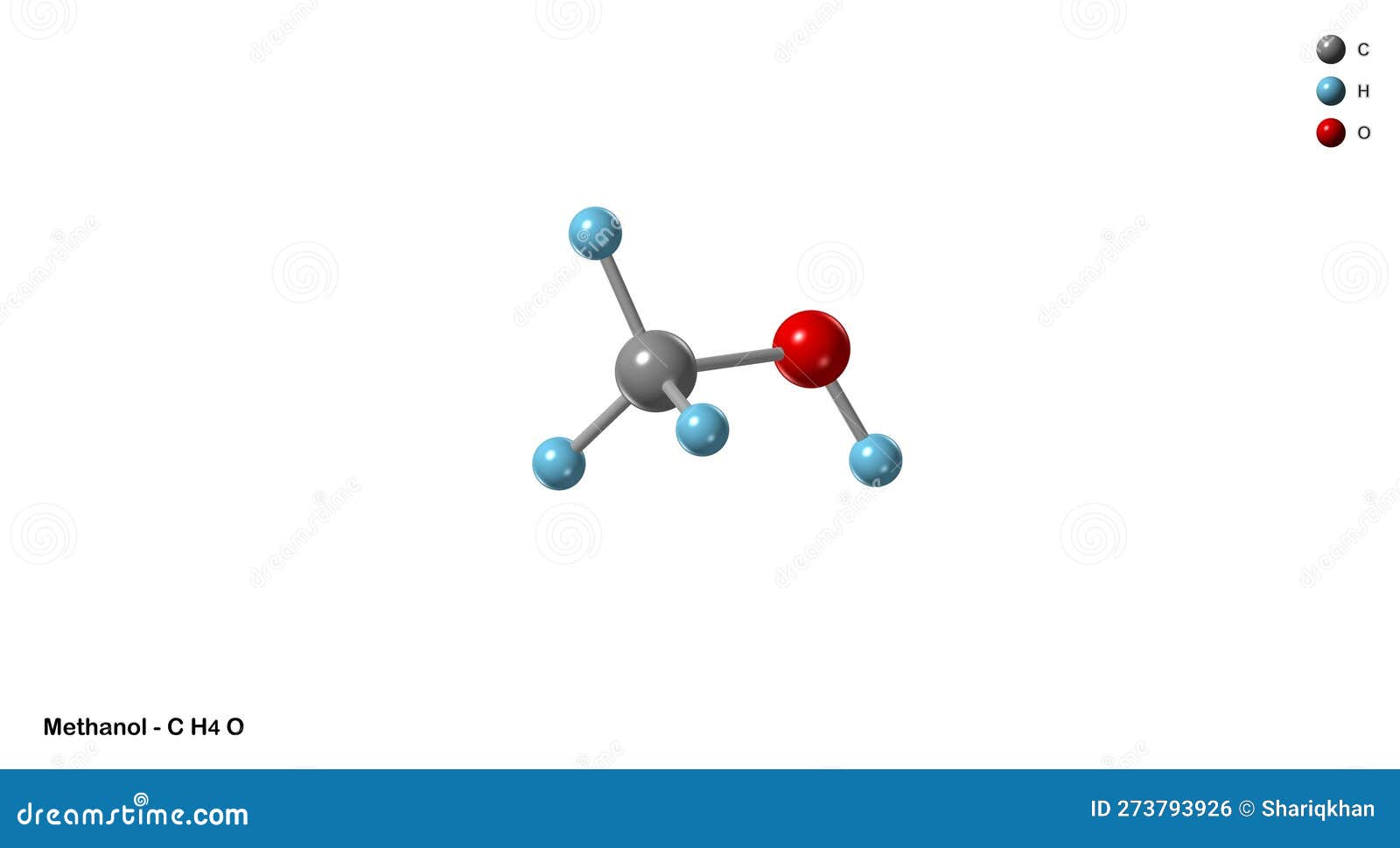
In the world of chemistry, understanding the Lewis structure of a molecule is crucial for predicting its properties and behavior. Methanol (CH3OH) is a simple yet fascinating molecule that serves as an excellent example for exploring Lewis dot structures. Let’s dive into the intricacies of the CH3OH Lewis structure, breaking it down step by step.
1. Introduction to Lewis Structures
Lewis structures, also known as electron dot diagrams, are visual representations of the distribution of valence electrons in a molecule. They help us understand how atoms bond and share electrons to achieve a stable electron configuration. For CH3OH, we’ll map out the arrangement of carbon ©, hydrogen (H), and oxygen (O) atoms and their shared electrons.
2. Step-by-Step Construction of CH3OH Lewis Structure
Step 1: Determine the Total Number of Valence Electrons
- Carbon ©: 4 valence electrons
- Hydrogen (H): 1 valence electron (×4 H atoms) = 4 electrons
- Oxygen (O): 6 valence electrons
- Total: 4 © + 4 (H) + 6 (O) = 14 valence electrons
Step 2: Identify the Central Atom
The central atom is typically the least electronegative element. In CH3OH, carbon © is the central atom, with oxygen (O) and hydrogen (H) atoms attached.
Step 3: Draw the Skeletal Structure
- Place C as the central atom.
- Attach O to C with a single bond.
- Attach three H atoms to C.
- Attach one H atom to O.
Skeletal structure: H3C-O-H
Step 4: Distribute Valence Electrons
- Start by forming single bonds between atoms (each bond uses 2 electrons).
- Remaining electrons are placed as lone pairs on the most electronegative atoms (O and H).
Bonding and Lone Pairs:
- C-O bond: 2 electrons
- Three C-H bonds: 6 electrons
- O-H bond: 2 electrons
- Remaining electrons: 4 (placed as lone pairs on O)
Step 5: Final Lewis Structure
The final Lewis structure of CH3OH is:
H H H
| | |
H - C - O - H
- Oxygen has two lone pairs (4 electrons).
- All atoms satisfy the octet rule (except H, which has a duet).
3. Formal Charge Analysis
Formal charge ensures the stability of the structure. Calculate it using:
Formal Charge = Valence Electrons - Lone Pairs - (Bonding Electrons / 2)
Formal Charges in CH3OH:
- C: 4 - 0 - (8 / 2) = 0
- O: 6 - 4 - (4 / 2) = 0
- H (attached to C): 1 - 0 - (2 / 2) = 0
- H (attached to O): 1 - 0 - (2 / 2) = 0
The presence of lone pairs on the oxygen atom causes a slight deviation from ideal tetrahedral angles, leading to a bent shape around the O-H bond.
5. Polarity of CH3OH
Methanol is a polar molecule due to:
- The O-H bond is highly polar (oxygen is more electronegative than hydrogen).
- The molecule has a net dipole moment due to the asymmetric arrangement of polar bonds.
CH3OH's polarity makes it soluble in water and useful in various chemical processes, including fuel production and solvents.
6. Applications of Methanol
Methanol is a versatile compound with applications in:
- Fuel: Used as an alternative to gasoline.
- Solvent: Widely used in laboratories and industries.
- Chemical Feedstock: Precursor for producing formaldehyde, acetic acid, and more.
"Methanol’s simple structure belies its importance in modern chemistry and industry."
7. Frequently Asked Questions (FAQ)
What is the hybridization of the carbon atom in CH3OH?
+The carbon atom in CH3OH is sp³ hybridized, forming four sigma bonds with hydrogen and oxygen atoms.
Why does CH3OH have a bent shape around the O-H bond?
+The lone pairs on the oxygen atom repel the bonding pairs, causing the O-H bond to bend and reducing the bond angle from 109.5° to ~104.5°.
Is CH3OH an acidic or basic molecule?
+CH3OH is a weak acid due to the polar O-H bond, which can donate a proton (H⁺) in aqueous solutions.
How does the Lewis structure of CH3OH relate to its solubility in water?
+The polar O-H bond in CH3OH allows it to form hydrogen bonds with water molecules, making it highly soluble in water.
8. Conclusion
The Lewis structure of CH3OH provides a foundation for understanding its molecular geometry, polarity, and reactivity. By following systematic steps—counting valence electrons, arranging atoms, and distributing electrons—we can accurately represent this molecule. Methanol’s simplicity and versatility make it a cornerstone in both academic and industrial chemistry.
Mastering Lewis structures like CH3OH enhances your ability to predict molecular behavior and apply chemical principles in real-world scenarios.


