Half Equivalence Point
Understanding the Half Equivalence Point in Acid-Base Titrations
In the world of analytical chemistry, titration is a cornerstone technique for determining the concentration of a substance in solution. Among the various stages of a titration, the half equivalence point holds particular significance, especially in acid-base titrations. This point is not just a milestone in the titration process but also a critical juncture for calculating the p*K*a (acid dissociation constant) of a weak acid or the p*K*b of a weak base. Let’s delve into what the half equivalence point is, why it matters, and how it’s calculated.
What is the Half Equivalence Point?
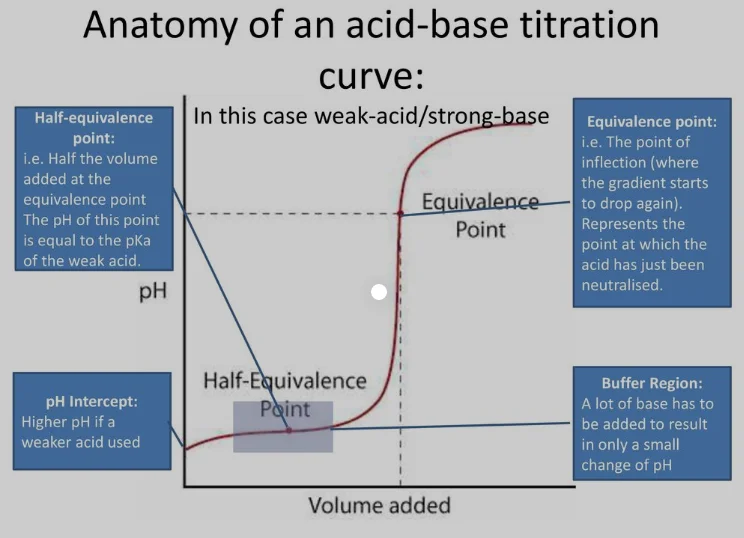
The half equivalence point occurs during a titration when the volume of the titrant added is exactly half of the volume required to reach the equivalence point. At this stage, the moles of the titrant (e.g., a strong base) added are equal to half the moles of the analyte (e.g., a weak acid). For acid-base titrations, this point is particularly important because it corresponds to the pH at which the concentration of the weak acid equals the concentration of its conjugate base.
Why is the Half Equivalence Point Important?
Determining p*K*a Values:
At the half equivalence point, the pH of the solution is numerically equal to the p*K*a of the weak acid. This relationship is derived from the Henderson-Hasselbalch equation, which describes the pH of a buffer solution:
[ \text{pH} = \text{p}K_a + \log\left(\frac{[\text{A}^-]}{[\text{HA}]}\right) ]
At the half equivalence point, the concentrations of the weak acid (HA) and its conjugate base (A^-) are equal, making the log term zero. Thus:
[ \text{pH} = \text{p}K_a ]Buffer Region Identification:
The half equivalence point lies within the buffer region of the titration curve, where the solution acts as an effective buffer. This region is characterized by a relatively flat pH curve, as the solution resists significant pH changes upon addition of small amounts of acid or base.Practical Applications:
Knowledge of the p*K*a is crucial in fields such as pharmacology (drug design), environmental science (acid-base chemistry of natural waters), and biochemistry (enzyme activity and protein structure).
How to Identify the Half Equivalence Point
The half equivalence point can be identified experimentally through the following methods:
pH Measurement:
Plotting the pH versus the volume of titrant added yields a titration curve. The half equivalence point is the volume at which the pH equals the p*K*a of the weak acid.Indicator Use:
While pH indicators are commonly used to detect the equivalence point, they are less reliable for pinpointing the half equivalence point. However, indicators with p*K*a values close to the expected p*K*a of the analyte can provide a visual approximation.Graphical Analysis:
The titration curve exhibits a distinct inflection point at the half equivalence point, where the slope of the curve is steepest. This inflection can be identified by examining the first derivative of the pH versus volume plot.
Example: Half Equivalence Point in Action
Consider the titration of acetic acid (CH₃COOH, p*K*a = 4.76) with sodium hydroxide (NaOH). At the half equivalence point:
- The moles of NaOH added equal half the moles of acetic acid initially present.
- The pH of the solution is 4.76, equal to the p*K*a of acetic acid.
- The solution contains equal concentrations of acetic acid and its conjugate base, acetate (CH₃COO^-), forming an effective buffer.
Comparative Analysis: Half vs. Equivalence Point
| Parameter | Half Equivalence Point | Equivalence Point |
|---|---|---|
| Definition | Volume of titrant = ½ of equivalence point volume | Volume of titrant = stoichiometrically equivalent to analyte |
| pH (for weak acid) | pH = p*K*a | pH > 7 (due to excess OH^- from strong base) |
| Significance | Determines p*K*a; buffer region | Complete neutralization; endpoint detection |

Practical Tips for Accurate Half Equivalence Point Determination
- Use a pH Meter: For precise pH measurements, especially in the buffer region.
- Stir Continuously: Ensure thorough mixing of the titrant and analyte to achieve equilibrium.
- Record Data Carefully: Small errors in volume or pH measurements can significantly affect results.
- Choose the Right Indicator: If using an indicator, select one with a p*K*a close to the expected p*K*a of the analyte.
Frequently Asked Questions (FAQ)
What is the difference between the half equivalence point and the equivalence point?
+The half equivalence point occurs when half the analyte has reacted, and its pH equals the p*K*a. The equivalence point occurs when all the analyte has reacted, and the pH depends on the excess titrant.
Why is the pH at the half equivalence point equal to the p*K*a?
+At this point, the concentrations of the weak acid and its conjugate base are equal, making the log term in the Henderson-Hasselbalch equation zero, so pH = p*K*a.
Can the half equivalence point be determined without a pH meter?
+While a pH meter is ideal, the half equivalence point can be approximated using a pH indicator with a p*K*a close to the analyte's p*K*a or by graphical analysis of the titration curve.
What happens if the titration is not performed accurately?
+Inaccurate titration can lead to incorrect determination of the half equivalence point and p*K*a, affecting the reliability of the results.
Conclusion
The half equivalence point is a critical concept in acid-base titrations, offering a direct method for determining the p*K*a of weak acids or p*K*b of weak bases. Its identification relies on understanding the principles of buffer solutions, the Henderson-Hasselbalch equation, and precise experimental techniques. By mastering this concept, chemists can unlock deeper insights into the behavior of acids and bases, enabling applications across diverse scientific and industrial fields.
Final Thought: The half equivalence point is not just a theoretical milestone—it’s a practical tool for unlocking the acid-base properties of compounds, bridging the gap between theory and real-world applications.



