Of2 Lewis Structure
Understanding the Lewis Structure of OF₂ (Oxygen Difluoride)
Oxygen difluoride (OF₂) is a fascinating molecule with unique bonding characteristics that make it an intriguing subject in chemical studies. Its Lewis structure provides critical insights into its molecular geometry, reactivity, and properties. Below, we break down the process of drawing the Lewis structure of OF₂, analyze its implications, and explore its significance in chemistry.
Step-by-Step Guide to Drawing the OF₂ Lewis Structure
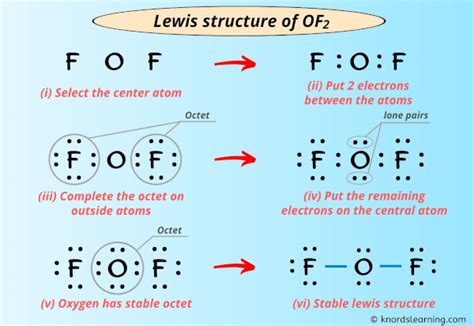
Determine the Total Number of Valence Electrons
- Oxygen (O) has 6 valence electrons.
- Each Fluorine (F) atom has 7 valence electrons.
- Total valence electrons = 6 (O) + 7 (F) + 7 (F) = 20 electrons.
- Oxygen (O) has 6 valence electrons.
Identify the Central Atom
- Oxygen (O) is the central atom due to its lower electronegativity compared to fluorine.
- Oxygen (O) is the central atom due to its lower electronegativity compared to fluorine.
Arrange the Atoms
- Place oxygen in the center and fluorine atoms on either side.
- Place oxygen in the center and fluorine atoms on either side.
Form Single Bonds
- Connect oxygen to each fluorine atom with a single bond, using 4 electrons (2 bonds).
- Connect oxygen to each fluorine atom with a single bond, using 4 electrons (2 bonds).
Complete the Octets
- Each fluorine atom requires 8 electrons to complete its octet. Place 6 electrons (3 lone pairs) around each fluorine.
- Oxygen will have 4 electrons remaining after bonding. These form 2 lone pairs on the oxygen atom.
- Each fluorine atom requires 8 electrons to complete its octet. Place 6 electrons (3 lone pairs) around each fluorine.
Verify the Structure
- Total electrons used: 4 (bonds) + 12 (F lone pairs) + 4 (O lone pairs) = 20 electrons, matching the total valence electrons.
- Total electrons used: 4 (bonds) + 12 (F lone pairs) + 4 (O lone pairs) = 20 electrons, matching the total valence electrons.
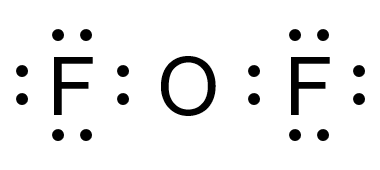
Final Lewis Structure:
F
│
O
│
F
- Oxygen has 2 lone pairs and 2 bonding pairs.
- Each fluorine has 3 lone pairs and 1 bonding pair.
Molecular Geometry and Bonding in OF₂
The Lewis structure of OF₂ reveals its V-shaped (bent) molecular geometry. This is due to the repulsion between the lone pairs on the oxygen atom and the bonding pairs, resulting in a bond angle slightly less than 109.5° (the tetrahedral angle).
Bonding Analysis:
- The O-F bonds are polar due to the electronegativity difference between oxygen (3.44) and fluorine (3.98).
- However, the molecule as a whole is polar because the bent shape does not cancel out the bond dipoles.
Key Properties of OF₂
- Physical State: OF₂ is a colorless, toxic gas at room temperature.
- Reactivity: It is a strong oxidizing agent and reacts violently with reducing agents.
- Applications: Used in rocket propellants and as a fluorinating agent in organic synthesis.
Comparative Analysis: OF₂ vs. Other Oxygen Fluorides
| Molecule | Lewis Structure | Geometry | Polarity |
|---|---|---|---|
| OF₂ | O with 2 lone pairs, 2 F atoms | Bent | Polar |
| O₂F₂ | O=O with 2 F atoms | Cis or Trans | Depends on isomer |
| F₂O | Similar to OF₂ but less stable | Bent | Polar |
OF₂ stands out due to its stability and distinct V-shaped geometry, making it a more commonly studied oxygen fluoride.
Practical Applications and Safety Considerations
OF₂’s oxidizing nature makes it valuable in specialized industries, but its toxicity and reactivity require stringent safety measures:
- Handling: Use in fume hoods with proper ventilation.
- Storage: Keep away from flammable materials and reducing agents.
FAQ Section
Why does OF₂ have a bent shape?
+The bent shape arises from the lone pairs on the oxygen atom repelling the bonding pairs, reducing the bond angle from the ideal tetrahedral angle.
Is OF₂ ionic or covalent?
+OF₂ is covalent because the bonds are formed by sharing electrons between oxygen and fluorine atoms.
How does OF₂ differ from water (H₂O) in terms of structure?
+Both have a bent shape, but OF₂ has 2 lone pairs on oxygen, while H₂O has 2 lone pairs and 2 hydrogen atoms. The bond angles differ slightly due to fluorine's larger size.
Why is OF₂ polar despite having polar bonds?
+The molecule is polar because the bent geometry prevents the bond dipoles from canceling each other out, resulting in a net molecular dipole.
Conclusion
The Lewis structure of OF₂ is a powerful tool for understanding its molecular behavior and properties. Its bent geometry, polar nature, and strong oxidizing capabilities make it a unique and important compound in chemistry. By mastering its structure, chemists can better predict its reactivity and applications in various fields.
OF₂’s Lewis structure highlights the importance of lone pairs in determining molecular geometry and polarity, making it a key example in VSEPR theory.